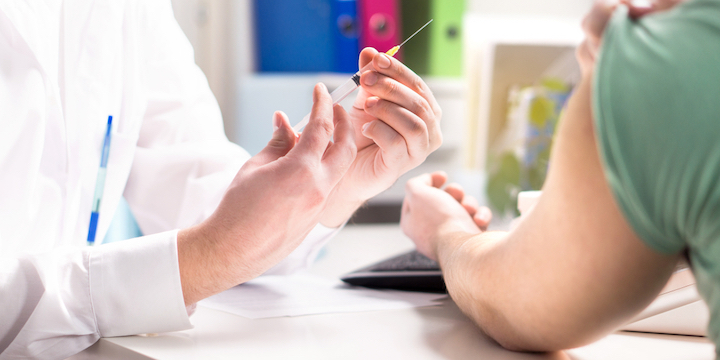
Vaccines for participants in the placebo group? Regulators, experts and laboratories are now faced with an ethical dilemma: when will participants in clinical trials of covid vaccines who have received a placebo be able to benefit from the real vaccine?
A simple saline solution
The question already arises for the vaccine from Pfizer and BioNTech, with which the vaccination campaign started in the United Kingdom and which should be authorized in the coming days in the United States.
The clinical trial conducted on this vaccine has recruited since late July 44,000 people in several countries, including the United States. Half received a placebo, a simple saline solution, and the other half the vaccine. The participants were placed in one or the other of the two groups in a random and “blind” fashion, that is, they did not know which group they belong to.
The usefulness of the placebo is to have a control group. Scientists can thus compare the number of cases of covid and the possible side effects between the two groups.
Read also : Covid vaccines: infecting volunteers, an ethical dilemma
General interest or special interest?
But today, should we promote the general interest or the particular interest of the participants? In the first case, the blind trial should be continued as long as possible in order to accumulate as much data as possible.
But in the second case, participants should be allowed to be vaccinated. Especially since those in charge of the trials cannot demand absolute altruism from them and many volunteers consider that they have the right to be vaccinated as a priority as soon as a vaccine is deemed safe and effective.
Wait for “their turn”
For Steven Goodman, an expert in epidemiology and population health who spoke to the advisory committee of the American Medicines Agency (FDA), the participants of a trial do not have an absolute right to be vaccinated before whether it be “their turn“in the rest of the population. It’s part of the contract.
He therefore suggests that participants be informed of what they received in the arm on the day the vaccine is authorized for the category of the population to which they belong. Placebo people could then ask to be vaccinated immediately.
Useful data, even after vaccination
This method has the advantage of encouraging participants to stay in the trial: they would thus continue to provide important data on the side effects and the effectiveness of the product. They would not have an interest in resigning from the trial to try to get vaccinated independently.
But in reality, nothing will prevent a 65-year-old from going to the pharmacy the day the vaccine is available there to his neighbors of the same age.
However, even when the placebo-control group wears off, the trial will continue to produce useful data on participants’ immune response and side effects.
Of course, the comparison between the control group and the vaccinated group will be impossible over several years, as is usually the case, because the development times for anti-covid vaccines have been drastically reduced. But the data of the participants vaccinated a few months before those of the control group will still make it possible to follow the possible appearance of side effects.
Which volunteers for the next trials?
On the other hand, another puzzle will emerge: when one or more vaccines become available, how will the developers of the other experimental vaccines be able to recruit participants in their own clinical trials? Who will accept the 50% risk of receiving a placebo, when a safe and effective vaccine is already available in pharmacies?
At this stage, the only clinical trials still possible could be on populations for which no vaccine has yet been authorized, for example young children.
 Cherry tomatoes contaminated with salmonella: 92 sick and 1 dead
Cherry tomatoes contaminated with salmonella: 92 sick and 1 dead  A better coaching method can make a person grow
A better coaching method can make a person grow  What is the method to prevent diabetes in children?
What is the method to prevent diabetes in children?  What are the effective factors in causing stomach ulcers?
What are the effective factors in causing stomach ulcers?  Why do embarrassing memories seem to appear at night?
Why do embarrassing memories seem to appear at night?  The amazing link between SARS-CoV-2 infection and newly started diabetes
The amazing link between SARS-CoV-2 infection and newly started diabetes  WHO says monkey pox is not a global emergency right now
WHO says monkey pox is not a global emergency right now  Single cell RNA sequencing uncovers new mechanisms of heart disease
Single cell RNA sequencing uncovers new mechanisms of heart disease  Hepatitis of unknown origin: 3 new deaths and 228 cases worldwide
Hepatitis of unknown origin: 3 new deaths and 228 cases worldwide